

Written by Professor Karen Dwyer, Nephrologist, Clinical Director, Kidney Health Australia
After many years of a sparsely filled toolbox, there has been a flurry of new drugs approved, or in the pipeline, for the management of kidney disease. The emergence of these therapies is helping to shift the focus to kidney preservation as well as provide clinicians with options to manage complications as they emerge.
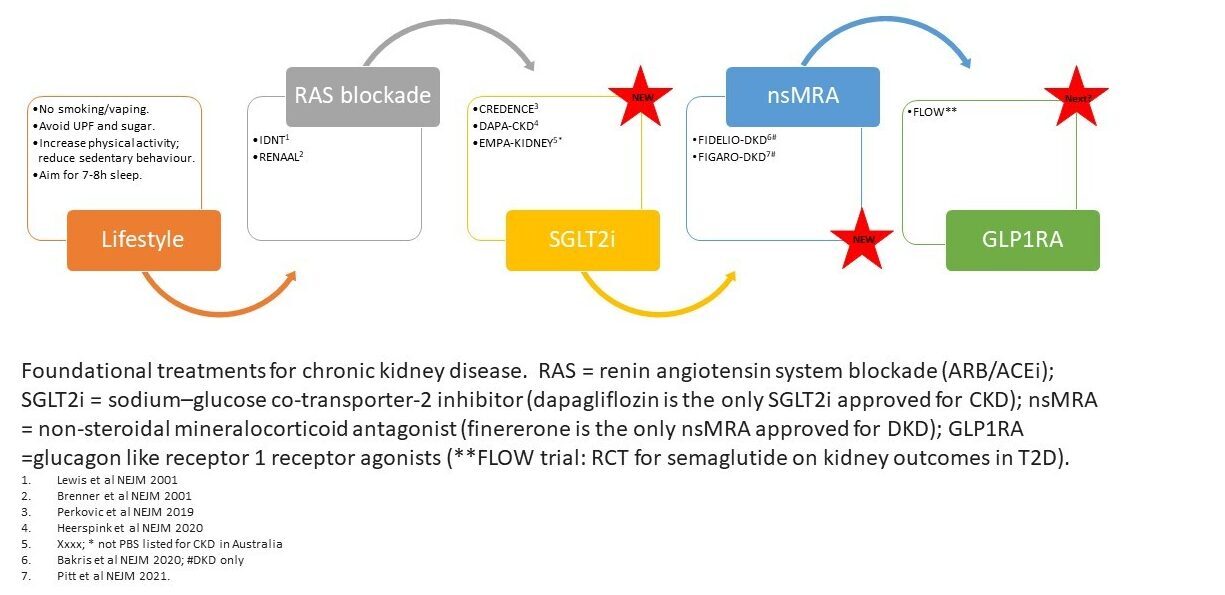
SGLT2i: RAS blockade with ACEi or ARBs has been the backbone in the management of CKD since the early 2000's. RAS blockade slows, but does not prevent, progression of kidney disease and is accepted as standard of care. Fast forward nearly 20 years and the introduction of the sodium glucose transporter 2 inhibitors (SGLT2i) into clinical practice has had a profound impact on the management of individuals with kidney disease and have quickly been incorporated into clinical guidelines. Indeed, SGLT2i are considered foundational therapy for people with chronic kidney disease (CKD) across a wide range of glomerular filtration rate and proteinuria levels, regardless of diabetes status or CKD aetiology.
Tips for using SGLT2i:
Sick Day Action Plan - see the 'How to Guide' and 'Sick Day Action Plan template' below.
nsMRA: non-steroidal mineralocorticoid antagonist, finererone has received PBS listing to delay progressive decline of kidney function and to reduce the risk of cardiovascular mortality and morbidity in adults with chronic kidney disease (with albuminuria) associated with Type 2 diabetes, in addition to standard of care (Authority streamline code 14097).
Tips for using nsMRA:
GLP1RA: The glucagon-like receptor 1 receptor agonists (GLP1RAs) are a class of molecules that were developed for the management of glycemic control in people with T2D. They improve glycemic control by stimulating insulin release in response to glucose load through incretin release. By delaying gastric emptying and effects on the satiety centre in the brain, they provoke weight loss. In people with albuminuria and T2D they reduce the albumin excretion rate. In addition to the remarkable weight loss provoked by GLP1RAs, these drugs reduce the risk of myocardial infarction. In a meta-analysis of 11 trials, it was demonstrated that GLP1RAs reduce the risk of myocardial infarctions by 11%. In contrast, no protection from heart failure is seen with these agents.
Tips for using GLP1RA:
CKD complications: Hyperkalaemia causes significant burden, and even mild hyperkalaemia has been independently associated with increased morbidity and mortality. Patients with chronic disease states, such as heart failure, hypertension, chronic kidney disease and diabetes mellitus, are increasingly susceptible to the development of hyperkalaemia. Patiromer has recently received PBS listing (Authority Streamline code 14327) for the treatment of chronic hyperkalemia (at least 2 episodes with K+>6mmol/L in the last 12 months) due to RAS blockade. Patiromer enhances potassium removal by exchanging calcium for potassium in the distal colon increasing faecal excretion of potassium.
Tips for Patiromer: The recommended starting dose is 8.4 g per day, which can be up titrated in 1 week or greater intervals by 8.4 g at a time to maximum dose 25.2 g per day.